Researchers participating in the Blueprint Neurotherapeutics Network receive grant funding (a UG3/UH3 award, or a U44 award if a small business) and no-cost access to drug development consultants and contract research organizations (CROs).
Research Grants (UG3/UH3 or U44)
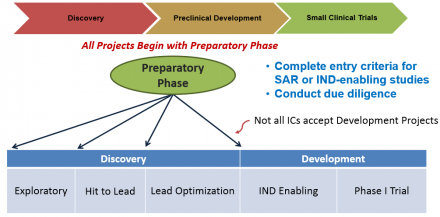
All projects that enter the BPN will begin with a preparatory phase of up to one year, which should be used to complete any studies required to initiate Discovery or Development activities and to engage the LDT in establishing a detailed research plan and go/no-go milestones for all subsequent work.
Projects can enter the BPN during the Discovery or Development stage and may seek support through phase I/first-in-human testing. Discovery involves iterative medicinal chemistry to improve the potency and ADMET properties of compounds in order to identify a development candidate. During Development, a development candidate undergoes preclinical toxicology testing required for an IND and manufacturing, ultimately advancing into phase I/first-in-human testing. BPN-supported Development activities include chemical manufacturing and controls (CMC), formulation development, toxicology studies, regulatory support, and phase I/first-in-human testing.
View all the Blueprint Neurotherapeutics funding opportunities.
External Oversight Committee (EOC)
BPN Consultants
Contract Research Organizations (CROs)
BPN Staff
Program Director
Charles Cywin, PhDHealth Program Specialist
Carolyn Bondar, PhDOperations Coordinator
Rakonda Medley, BS
